Bergmann's rule is an ecogeographical rule that states that within a broadly distributed taxonomic clade, populations and species of larger size are found in colder environments, while populations and species of smaller size are found in warmer regions. The rule derives from the relationship between size in linear dimensions meaning that both height and volume will increase in colder environments. Bergmann's rule only describes the overall size of the animals, but does not include body proportions like Allen's rule does.
Although originally formulated in relation to species within a genus, it has often been recast in relation to populations within a species. It is also often cast in relation to latitude. It is possible that the rule also applies to some plants, such as Rapicactus.
The rule is named after nineteenth century German biologist Carl Bergmann, who described the pattern in 1847, although he was not the first to notice it. Bergmann's rule is most often applied to mammals and birds which are endotherms, but some researchers have also found evidence for the rule in studies of ectothermic species,[2][3] such as the ant Leptothorax acervorum. While Bergmann's rule appears to hold true for many mammals and birds, there are exceptions.[4][5][6]
Larger-bodied animals tend to conform more closely to Bergmann's rule than smaller-bodied animals, at least up to certain latitudes. This perhaps reflects a reduced ability to avoid stressful environments, such as by burrowing.[7] In addition to being a general pattern across space, Bergmann's rule has been reported in populations over historical and evolutionary time when exposed to varying thermal regimes.[8][9][10] In particular, temporary, reversible dwarfing of mammals has been noted during two relatively brief upward excursions in temperature during the Paleogene: the Paleocene-Eocene thermal maximum[11] and the Eocene Thermal Maximum 2.[12]
Examples
Humans
Human populations near the poles, including the Inuit, Aleut, and Sami people, are on average heavier than populations from mid-latitudes, consistent with Bergmann's rule.[14] They also tend to have shorter limbs and broader trunks, consistent with Allen's rule.[14] According to Marshall T. Newman in 1953, Native American populations are generally consistent with Bergmann's rule although the cold climate and small body size combination of the Eastern Inuit, Canoe Nation, Yuki people, Andes natives and Harrison Lake Lillooet runs contrary to the expectations of Bergmann's rule.[15] Newman contends that Bergmann's rule holds for the populations of Eurasia, but it does not hold for those of sub-Saharan Africa.[15]
Human populations also show a decrease in stature with an increase in mean annual temperature.[16] Bergmann's rule holds for Africans with the pygmy phenotype and other pygmy peoples. These populations show a shorter stature and smaller body size due to an adaptation to hotter and more humid environments.[17] With elevated environmental humidity, evaporative cooling (sweating) is a less effective way to dissipate body heat, but a higher surface area to volume ratio should provide a slight advantage through passive convective heat loss.
Birds
A 2019 study of changes in the morphology of migratory birds used bodies of birds which had collided with buildings in Chicago from 1978 to 2016. The length of birds' lower leg bones (an indicator of body size) shortened by an average of 2.4% and their wings lengthened by 1.3%. A similar study published in 2021 used measurements of 77 nonmigratory bird species captured live for banding in lowland Amazon rainforest. Between 1979 and 2019, all study species have gotten smaller on average, by up to 2% per decade. The morphological changes are regarded as resulting from global warming, and may demonstrate an example of evolutionary change following Bergmann's rule.[18][19][20][21]
Reptiles
Bergmann's rule has been reported to be vaguely followed by female crocodilians.[22][23] However, for turtles[24] or lizards[25] the rule's validity has not been supported.
Invertebrates
Evidence of Bergmann's rule has been found in marine copepods.[26]
Plants
Bergmann's rule cannot generally be applied to plants.[27] Regarding Cactaceae, the case of the saguaro (Carnegiea gigantea), once described as "a botanical Bergmann trend",[28] has instead been shown to depend on rainfall, particularly winter precipitation, and not temperature.[29] Members of the genus Rapicactus are larger in cooler environments, as their stem diameter increases with altitude and particularly with latitude. However, since Rapicactus grow in a distributional area in which average precipitation tends to diminish at higher latitudes, and their body size is not conditioned by climatic variables, this could suggest a possible Bergmann trend.[30]
Explanations

The earliest explanation, given by Bergmann when originally formulating the rule, is that larger animals have a lower surface area to volume ratio than smaller animals, so they radiate less body heat per unit of mass, and therefore stay warmer in cold climates. Warmer climates impose the opposite problem: body heat generated by metabolism needs to be dissipated quickly rather than stored within.[31]
Thus, the higher surface area-to-volume ratio of smaller animals in hot and dry climates facilitates heat loss through the skin and helps cool the body. It is important to note that when analyzing Bergmann's Rule in the field that groups of populations being studied are of different thermal environments, and also have been separated long enough to genetically differentiate in response to these thermal conditions.[31] The relationship between stature and mean annual temperature can be explained by modeling any shape that is increasing in any dimension. As you increase the height of a shape, its surface area-to-volume ratio will decrease. Modeling a person's trunk and limbs as cylinders shows a 17% decrease in surface area-to-volume ratio from a person who is five feet tall to a person who is six feet tall even at the same body mass index (BMI).
In marine crustaceans, it has been proposed that an increase in size with latitude is observed because decreasing temperature results in increased cell size and increased life span, both of which lead to an increase in maximum body size (continued growth throughout life is characteristic of crustaceans).[3] The size trend has been observed in hyperiid and gammarid amphipods, copepods, stomatopods, mysids, and planktonic euphausiids, both in comparisons of related species as well as within widely distributed species.[3] Deep-sea gigantism is observed in some of the same groups, possibly for the same reasons.[3] An additional factor in aquatic species may be the greater dissolved oxygen concentration at lower temperature. This view is supported by the reduced size of crustaceans in high-altitude lakes.[32] A further possible influence on invertebrates is reduced predation pressure at high latitude.[33] A study of shallow water brachiopods found that predation was reduced in polar areas relative to temperate latitudes (the same trend was not found in deep water, where predation is also reduced, or in comparison of tropical and temperate brachiopods, perhaps because tropical brachiopods have evolved to smaller sizes to successfully evade predation).[33]
Hesse's rule
In 1937 German zoologist and ecologist Richard Hesse proposed an extension of Bergmann's rule. Hesse's rule, also known as the heart–weight rule, states that species inhabiting colder climates have a larger heart in relation to body weight than closely related species inhabiting warmer climates.[34]
Criticism
In a 1986 study, Valerius Geist claimed Bergmann's rule to be false: the correlation with temperature is spurious; instead, Geist found that body size is proportional to the duration of the annual productivity pulse, or food availability per animal during the growing season.[35]
Because many factors can affect body size, there are many critics of Bergmann's rule. Some believe that latitude itself is a poor predictor of body mass. Examples of other selective factors that may contribute to body mass changes are the size of food items available, effects of body size on success as a predator, effects of body size on vulnerability to predation, and resource availability. For example, if an organism is adapted to tolerate cold temperatures, it may also tolerate periods of food shortage, due to correlation between cold temperature and food scarcity.[5] A larger organism can rely on its greater fat stores to provide the energy needed for survival as well being able to procreate for longer periods.
Resource availability is a major constraint on the overall success of many organisms. Resource scarcity can limit the total number of organisms in a habitat, and over time can also cause organisms to adapt by becoming smaller in body size. Resource availability thus becomes a modifying restraint on Bergmann's Rule.[36]
See also
References
- ↑ FRYDRÝŠEK, Karel (2019). Biomechanika 1. Ostrava, Czech Republic: VSB – Technical University of Ostrava, Faculty of Mechanical Engineering, Department of Applied Mechanics. pp. 337–338. ISBN 978-80-248-4263-9.
- ↑ Olalla-Tárraga, Miguel Á.; Rodríguez, Miguel Á.; Hawkins, Bradford A. (2006). "Broad-scale patterns of body size in squamate reptiles of Europe and North America". Journal of Biogeography. 33 (5): 781–793. doi:10.1111/j.1365-2699.2006.01435.x. S2CID 59440368.
- 1 2 3 4 Timofeev, S. F. (2001). "Bergmann's Principle and Deep-Water Gigantism in Marine Crustaceans". Biology Bulletin of the Russian Academy of Sciences. 28 (6): 646–650. doi:10.1023/A:1012336823275. S2CID 28016098.
- ↑ Meiri, S.; Dayan, T. (2003-03-20). "On the validity of Bergmann's rule". Journal of Biogeography. 30 (3): 331–351. doi:10.1046/j.1365-2699.2003.00837.x. S2CID 11954818.
- 1 2 Ashton, Kyle G.; Tracy, Mark C.; Queiroz, Alan de (October 2000). "Is Bergmann's Rule Valid for Mammals?". The American Naturalist. 156 (4): 390–415. doi:10.1086/303400. JSTOR 10.1086/303400. PMID 29592141. S2CID 205983729.
- ↑ Millien, Virginie; Lyons, S. Kathleen; Olson, Link; et al. (May 23, 2006). "Ecotypic variation in the context of global climate change: Revisiting the rules". Ecology Letters. 9 (7): 853–869. doi:10.1111/j.1461-0248.2006.00928.x. PMID 16796576.
- ↑ Freckleton, Robert P.; Harvey, Paul H.; Pagel, Mark (2003). "Bergmann's rule and body size in mammals". The American Naturalist. 161 (5): 821–825. doi:10.1086/374346. JSTOR 10.1086/374346. PMID 12858287. S2CID 44612517.
- ↑ Smith, Felia A.; Betancourt, Julio L.; Brown, James H. (December 22, 1995). "Evolution of Body Size in the Woodrat over the Past 25,000 Years of Climate Change". Science. 270 (5244): 2012–2014. Bibcode:1995Sci...270.2012S. doi:10.1126/science.270.5244.2012. S2CID 129915445.
- ↑ Huey, Raymond B.; Gilchrist, George W.; Carlson, Margen L.; Berrigan, David; Serra, Luıs (January 14, 2000). "Rapid Evolution of a Geographic Cline in Size in an Introduced Fly". Science. 287 (5451): 308–309. Bibcode:2000Sci...287..308H. doi:10.1126/science.287.5451.308. PMID 10634786. S2CID 23209206.
- ↑ Hunt, Gene; Roy, Kaustuv (January 31, 2006). "Climate change, body size evolution, and Cope's rule in deep-sea ostracodes". Proceedings of the National Academy of Sciences of the United States of America. 103 (5): 1347–1352. Bibcode:2006PNAS..103.1347H. doi:10.1073/pnas.0510550103. PMC 1360587. PMID 16432187.
- ↑ Secord, R.; Bloch, J.I.; Chester, S.G.B.; Boyer, D.M.; Wood, A.R.; Wing, S.L.; Kraus, M.J.; McInerney, F.A.; Krigbaum, J. (2012). "Evolution of the Earliest Horses Driven by Climate Change in the Paleocene-Eocene Thermal Maximum". Science. 335 (6071): 959–962. Bibcode:2012Sci...335..959S. doi:10.1126/science.1213859. PMID 22363006. S2CID 4603597. Archived from the original on 2019-04-09. Retrieved 2020-01-08.
- ↑ Erickson, Jim (November 1, 2013). "Global warming led to dwarfism in mammals — twice". University of Michigan. Retrieved 2013-11-12.
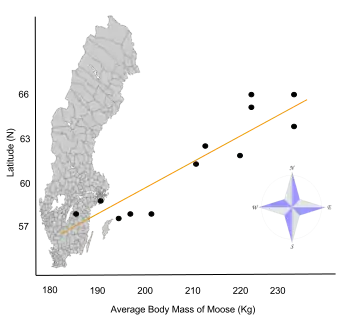
- ↑ Sand, Håkan K.; Cederlund, Göran R.; Danell, Kjell (June 1995). "Geographical and latitudinal variation in growth patterns and adult body size of Swedish moose (Alces alces)". Oecologia. 102 (4): 433–442. Bibcode:1995Oecol.102..433S. doi:10.1007/BF00341355. PMID 28306886. S2CID 5937734.
- 1 2 Holliday, Trenton W.; Hilton, Charles E. (June 2010). "Body proportions of circumpolar peoples as evidenced from skeletal data: Ipiutak and Tigara (Point Hope) versus Kodiak Island Inuit". American Journal of Physical Anthropology. 142 (2): 287–302. doi:10.1002/ajpa.21226. PMID 19927367.
- 1 2 Newman, Marshall T. (August 1953). "The Application of Ecological Rules to the Racial Anthropology of the Aboriginal New World". American Anthropologist. 55 (3): 311–327. doi:10.1525/aa.1953.55.3.02a00020.
- ↑ Roberts, DF (1954). "Body weight, race and climate". American Journal of Physical Anthropology. 11 (4): 533–558. doi:10.1002/ajpa.1330110404.
- ↑ Dominy, Nathaniel; Perry, George (February 25, 2009). "Evolution of the human pygmy phenotype".
- ↑ Vlamis, K. (4 December 2019). "Birds 'shrinking' as the climate warms". BBC News. Retrieved 5 December 2019.
- ↑ Liao, Kristine (4 December 2019). "North American Birds Are Shrinking, Likely a Result of the Warming Climate". Audubon. Retrieved 5 December 2019.
- ↑ Weeks, B. C.; Willard, D. E.; Zimova, M.; Ellis, A. A.; Witynski, M. L.; Hennen, M.; Winger, B. M. (2019). "Shared morphological consequences of global warming in North American migratory birds". Ecology Letters. 23 (2): 316–325. doi:10.1111/ele.13434. hdl:2027.42/153188. PMID 31800170. S2CID 208620935.
- ↑ Jirinec, Vitek; Burner, Ryan C.; Amaral, Bruna R.; BierregaardJr, Richard O.; Fernández-Arellano, Gilberto; Hernández-Palma, Angélica; Johnson, Erik I.; Lovejoy, Thomas E.; Powell, Luke L.; Rutt, Cameron L.; Wolfe, Jared D. (2021). "Morphological consequences of climate change for resident birds in intact Amazonian rainforest". Science Advances. 7 (46): eabk1743. Bibcode:2021SciA....7.1743J. doi:10.1126/sciadv.abk1743. PMC 8589309. PMID 34767440.
- ↑ Lakin, R.J.; Barrett, P.M.; Stevenson, C.; Thomas, R.J.; Wills, M.A. (2020). "First evidence for a latitudinal body mass effect in extant Crocodylia and the relationships of their reproductive characters". Biological Journal of the Linnean Society. 129 (4): 875–887. doi:10.1093/biolinnean/blz208.
- ↑ Georgiou, A. (12 March 2020). "Crocodilians, Which Have Walked Earth for Nearly 100 Million Years, Are Survivors of Mass Extinctions and May Be Able to Adapt to Climate Change". newsweek.com. Newsweek. Retrieved 2020-03-13.
- ↑ Angielczyk, K.D.; Burroughs, R.W.; Feldman, C.R. (2015). "Do turtles follow the rules? Latitudinal gradients in species richness, body size, and geographic range area of the world's turtles". Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 324 (3): 270–294. doi:10.1002/jez.b.22602. PMID 25588662.
- ↑ Pincheira-Donoso, D.; Hodgson, D.J.; Tregenza, T. (2008). "The evolution of body size under environmental gradients in ectotherms: why should Bergmann's rule apply to lizards?". BMC Evolutionary Biology. 8 (68): 68. doi:10.1186/1471-2148-8-68. PMC 2268677. PMID 18304333.
- ↑ Campbell, M.D.; et al. (2021-08-21). "Testing Bergmann's Rule in marine copepods". Ecography. 44 (9): 1283–1295. doi:10.1111/ecog.05545. hdl:10072/407178. S2CID 238701490.
- ↑ Moles, A. T.; Warton, D. I.; Warman, L.; Swenson, N. G.; Laffan, S. W.; Zanne, A. E.; Pitman, A.; Hemmings, F. A.; Leishman, M. R. (2009-09-01). "Global patterns in plant height". Journal of Ecology. 97 (5): 923–932. doi:10.1111/j.1365-2745.2009.01526.x.
- ↑ Niering, W.A.; Whittaker, R.H.; Lowe, C.H. (1963). "The saguaro: a population in relation to environment". Science. 142 (3588): 15–23. Bibcode:1963Sci...142...15N. doi:10.1126/science.142.3588.15. PMID 17812501.
- ↑ Drezner, T. D. (2003-03-01). "Revisiting Bergmann's rule for saguaros (Carnegiea gigantea (Engelm.) Britt. and Rose): stem diameter patterns over space". Journal of Biogeography. 30 (3): 353–359. doi:10.1046/j.1365-2699.2003.00834.x. S2CID 82276407.
- ↑ Donati, D.; Bianchi, C.; Pezzi, G.; Conte, L.; Hofer, A.; Chiarucci, A. (2016). "Biogeography and ecology of the genus Turbinicarpus (Cactaceae): environmental controls of taxa richness and morphology". Systematics and Biodiversity. 15 (4): 361–371. doi:10.1080/14772000.2016.1251504. S2CID 90330480.
- 1 2 Brown, James H.; Lee, Anthony K. (January 1969). "Bergmann's Rule and Climatic Adaptation in Woodrats (Neotoma)". Evolution. 23 (2): 329–338. doi:10.2307/2406795. JSTOR 2406795. PMID 28562890.
- ↑ Peck, L. S.; Chapelle, G. (2003). "Reduced oxygen at high altitude limits maximum size". Proceedings of the Royal Society of London. Series B: Biological Sciences. 270 (suppl. 2): S166–S167. doi:10.1098/rsbl.2003.0054. PMC 1809933. PMID 14667371.
- 1 2 Harper, E. M.; Peck, L. S. (2016). "Latitudinal and depth gradients in marine predation pressure". Global Ecology and Biogeography. 25 (6): 670–678. doi:10.1111/geb.12444.
- ↑ Baum, Steven (January 20, 1997). "Hesse's rule". Glossary of Oceanography and the Related Geosciences with References. Texas Center for Climate Studies, Texas A&M University. Archived from the original on December 22, 2010. Retrieved 2011-01-09.
- ↑ Geist, Valerius (April 1987). "Bergmann's rule is invalid". Canadian Journal of Zoology. 65 (4): 1035–1038. doi:10.1139/z87-164.
- ↑ Clauss, Marcus; Dittmann, Marei T.; Müller, Dennis W. H.; et al. (October 2013). "Bergmann′s rule in mammals: A cross-species interspecific pattern" (PDF). Oikos. 122 (10): 1465–1472. doi:10.1111/j.1600-0706.2013.00463.x. S2CID 44183222.
Notes
- Bergmann, Carl (1847). "Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse". Göttinger Studien. 3 (1): 595–708.
- Roberts DF (1953). "Body weight, race and climate". American Journal of Physical Anthropology. 11 (4): 533–558. doi:10.1002/ajpa.1330110404. PMID 13124471.
- Roberts DF (1978). Climate and Human Variability (2nd ed.). Menlo Park, CA: Cummings. ISBN 9780846566250.
- Ruff CB (1994). "Morphological adaptation to climate in modern and fossil hominids". Yearbook of Physical Anthropology. 37: 65–107. doi:10.1002/ajpa.1330370605.
- Schreider E (1950). "Geographical distribution of the body-weight/body-surface ratio". Nature. 165 (4190): 286. Bibcode:1950Natur.165..286S. doi:10.1038/165286b0. PMID 15410342.