 | |
| Names | |
|---|---|
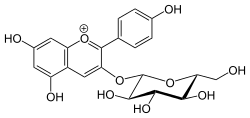
| IUPAC name
5,7‐Dihydroxy‐2‐(4‐hydroxyphenyl)‐3‐{[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy}‐1λ4‐chromen‐1‐ylium | |
| Systematic IUPAC name
5,7-Dihydroxy-2-(4-hydroxyphenyl)-3-chromeniumyl β-D-glucopyranoside | |
| Other names
Pelargonidin-3-O-glucoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C21H21O10+ | |
| Molar mass | 433.38 g/mol |
| UV-vis (λmax) | 505 nm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Callistephin is an anthocyanin. It is the 3-O-glucoside of pelargonidin.
It is found in pomegranate juice,[2] in strawberries[3] and in purple corn.[4] It is also found in the berry skins of Cabernet Sauvignon and Pinot Noir grapes (Vitis vinifera L.).[5]
See also
References
- ↑ Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Fei He, Na-Na Liang, Lin Mu, Qiu-Hong Pan, Jun Wang, Malcolm J. Reeves and Chang-Qing Duan, Molecules, 2012, 17, pages 1571-1601, doi:10.3390/molecules17021571
- ↑ Evolution of juice anthocyanins during ripening of new selected pomegranate (Punica granatum) clones. F. Hernández, P. Melgarejo, F. A. Tomás-Barberán and F. Artés, European Food Research and Technology, November 1999, Volume 210, Issue 1, pages 39-42, doi:10.1007/s002170050529
- ↑ Mullen, William; Edwards, Christine A.; Serafini, Mauro; Crozier, Alan (2008). "Bioavailability of Pelargonidin-3-O-glucoside and Its Metabolites in Humans Following the Ingestion of Strawberries with and without Cream". Journal of Agricultural and Food Chemistry. 56 (3): 713–9. doi:10.1021/jf072000p. PMID 18211024.
- ↑ Anthocyanins isolated from purple corn (Zea mays L.). Hiromitsu Aoki, Noriko Kuze and Yoshiaki Kato (article Archived 2013-10-29 at the Wayback Machine)
- ↑ Mass-spectrometry evidence confirming the presence of pelargonidin-3-O-glucoside in the berry skins of Cabernet Sauvignon and Pinot Noir (Vitis vinifera L.). F. HE, J.-J. HE, Q.-H. PAN and C.-Q. DUAN, Australian Journal of Grape and Wine Research, October 2010, Volume 16, Issue 3, pages 464–468, doi:10.1111/j.1755-0238.2010.00107.x
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.