An Evans balance, also known as a Johnson-Matthey balance or a magnetic susceptibility balance (MSB), is a device for measuring the magnetic susceptibility of a sample. Magnetic susceptibility is related to the force experienced by a substance in a magnetic field. Various practical devices are available, differing in the shape of the magnetic field and the way the force is measured.[1]
Unlike the traditional Gouy balance, which directly measures the force on the sample in a magnetic field, an Evans balance measures the force on the magnet.[2]
Mechanism
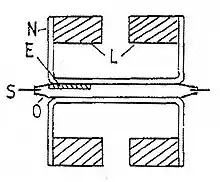
The suspension trip has two pairs of magnets placed back-to-back, making a balanced system with a magnetic field at each end. When a sample, which is fixed in a glass tube holder, is introduced into the field of one magnet, it experiences a force that deflects the beam. The deflection is detected by an optical transducer. A magnetic field is generated at the second magnet which, by negative feedback, restores the beam to its original position. The magnetic field is generated by passing a current through a coil of wire. One end of this wire is between the poles of the second magnet. The current required to do this is proportional to the force exerted on the first magnet. There is a second coil of wire that creates an electrical zero and a copper sheet that can critically dampen the system.[3]
The original Evans balance was described by the English scientist Dennis F. Evans in 1973, based on a torsional balance developed in 1937 by Alexander Rankine. Evans used Ticonal bars with cadmium-plated mild steel yokes as the magnets and a Johnson Matthey gold alloy (hence the other name of the balance) for the suspension strip, glued together with epoxy resin onto a phosphor brown spacer. The tubes were made from NMR tubes and the current came from CdS photocells.[3] This original was modified with help from the Johnson Matthey company. Two pairs of magnets were glued between the arms of an H-frame. The sample was placed into the gap between one pair of magnets and a small coil in the gap between the second pair of magnets. This entire construction pivoted horizontally around a torsion strip. When a sample tube was placed between the first pair of magnets, the torsional force was restored by the current passed through the coil between the second pair of magnets, giving a reading on a display instead of a Helipot potentiometer (as was used in the original).[4]
Comparison to alternative magnetic balances
The main advantage of this system is that it is cheap to construct, as it does not require a precision weighing device. Using an Evans balance is less time-consuming than using Gouy or Faraday balances, although it is not as sensitive or accurate in comparison.[3] An Evans balance has a range from 0.001 x 10−7 to 1.99 x 10−7 CGS volume susceptibility units.[5] The original Evans balance had an accuracy within 1% of literature values for diamagnetic solutions and within 2% of literature values for paramagnetic solids.[3]
The system allows for measurements of solid, liquid, and gaseous forms of a wide range of paramagnetic and diamagnetic materials. For each measurement, around 250 mg of sample is required (50 mg can be used for a thin-bore sample tube).[6]
Calibration
The Evans balance measures susceptibility indirectly by referring to a calibration standard of known susceptibility. The most convenient compound for this purpose is mercury cobalt thiocyanate, HgCo(NCS)4, which has a susceptibility of 16.44×10−6 (±0.5%) CGS at 20 °C.[7] Another common calibration standard is [Ni(en)3]S2O3 which has a susceptibility of 1.104 x 10−5 erg G−2 cm−3.[8] Three readings of the meter are needed: of an empty tube, R0; of the tube filled with calibrant; and of the tube filled with the sample, Rs. Some balances have an auto-tare feature that eliminates the need for the R0 measurement.[9] Accuracy depends somewhat on the homogeneous packing of the sample. The first two provide a calibration constant, C. The mass susceptibility in grams is calculated as
where L is the length of the sample, C is the calibration constant (usually 1 if it has been calibrated), and m is its mass in grams. The reading for the empty tube is needed because the tube glass is diamagnetic. There is a V term multiplied by an A term in the most general form of the equation. These two terms (V∗A) are collectively added to the numerator in the above equation. The V term is the volume susceptibility of air (0.029 x 10−6 erg G−2 cm−3) and A is the cross-sectional area of the sample. These two terms can be ignored for solid samples, yielding the original equation written above.[8]
To calculate the volume magnetic susceptibility (χ) instead of the weight susceptibility (χg), such as in a liquid sample, the equation would have the extra V term added to the numerator and instead of being divided by m, the equation would be divided by d for the density of the solution.[3]
References
- ↑ O'Connor, C.J. (1982). Lippard, S.J. (ed.). Magnetic susceptibility measurements. Progress in Inorganic Chemistry. Vol. 29. Wiley. p. 203. ISBN 978-0-470-16680-2.
- ↑ "Illustration of commercial Evans balance". Archived from the original on 2011-07-16. Retrieved 2011-02-19.
- 1 2 3 4 5 Evans, D.F. (1974). "A new type of magnetic balance". Journal of Physics E: Scientific Instruments. 7 (4): 247. Bibcode:1974JPhE....7..247E. doi:10.1088/0022-3735/7/4/007.
- ↑ "Classic Kit: The Evans balance". Retrieved 5 September 2023.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2014-10-29. Retrieved 2014-10-29.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2014-10-29. Retrieved 2014-10-29.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Figgis, B.N.; Lewis, J. (1960). "The Magnetochemistry of Complex Compounds". In Lewis. J. and Wilkins. R.G. (ed.). Modern Coordination Chemistry. New York: Wiley. p. 415
- 1 2 http://alpha.chem.umb.edu/chemistry/ch371/documents/MicroscaleDeterminationofMagneticSusceptibility_001.pdf
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2014-10-29. Retrieved 2014-10-29.
{{cite web}}: CS1 maint: archived copy as title (link)