 | |
| Names | |
|---|---|
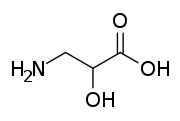
| IUPAC name
3-Amino-2-hydroxypropanoic acid | |
| Other names
3-Aminolactic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C3H7NO3 | |
| Molar mass | 105.093 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Isoserine is a non-proteinogenic α-hydroxy-β-amino acid, and an isomer of serine. Non-proteinogenic amino acids do not form proteins, and are not part of the genetic code of any known organism. Isoserine has only been produced synthetically.
The first documented synthesis of isoserine in a laboratory setting was by Miyazawa et al., who published their results in 1976.[1]
See also
References
- ↑ Ziora, Zyta; Skwarcynski, Mariusz; Kiso, Yoshiaki (2011). "Medicinal Chemistry of α-Hydroxy-β-Amino Acids". In Hughes, Andrew B. (ed.). Amino Acids, Peptides and Proteins in Organic Chemistry, Volume 4: Protection, Reactions, Medicinal Chemistry, Combinatorial Synthesis. Wiley-VCH. Section 6.2.2: Synthesis of α-Hydroxy-β-amino acids. ISBN 978-3-527-63182-7. OCLC 741558720. Retrieved 2017-06-10 – via Google Books.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.