 | |
 | |
| Names | |
|---|---|
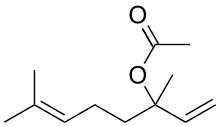
| IUPAC name
3,7-Dimethylocta-1,6-dien-3-yl acetate | |
| Other names
Bergamiol Bergamol Linalool acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.743 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H20O2 | |
| Molar mass | 196.290 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.895 g/cm3 |
| Boiling point | 220 °C (428 °F; 493 K) |
| Insoluble | |
| Solubility in organic solvents | Soluble |
| Hazards | |
| Flash point | 69.6 °C (157.3 °F; 342.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Linalyl acetate is an organic compound, the acetate ester of linalool and a phytochemical found in many flowers and spice plants. It is one of the principal components of the essential oils of bergamot and lavender.[1] It often occurs together with linalool and is a widely used fragrance. [2]
The chemical tastes similar to how it smells with a pleasant fruity odor reminiscent of bergamot mint oil. It is found in Eau de Cologne mint and is mildly toxic to humans, toxic to fish, and extremely toxic to daphnia. Linalyl acetate is also combustible.
Safety
Linalyl acetate is found safe as a fragrance material under current levels of use.[3]
See also
References
- 1 2 The Merck Index, 15th Ed. (2013), p. 1022, Monograph 5551, O'Neil: The Royal Society of Chemistry. Available online at: http://www.rsc.org/Merck-Index/monograph/mono1500005551
- ↑ Sell, Charles S. (2006). "Terpenoids". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.2005181602120504.a01.pub2. ISBN 0471238961.
- ↑ "RIFM fragrance ingredient safety assessment, Linalyl acetate, CAS Registry Number 115-95-7" (PDF). Food and Chemical Toxicology. 82: S39–S48. 2015.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.