
The upstream signaling pathway is triggered by the binding of a signaling molecule, a ligand, to a receiving molecule, a receptor. Receptors and ligands exist in many different forms, and only recognize/bond to particular molecules. Upstream extracellular signaling transduce a variety of intracellular cascades.[1]
Receptors and ligands are common upstream signaling molecules that dictate the downstream elements of the signal pathway. A plethora of different factors affect which ligands bind to which receptors and the downstream cellular response that they initiate.
TGF-β
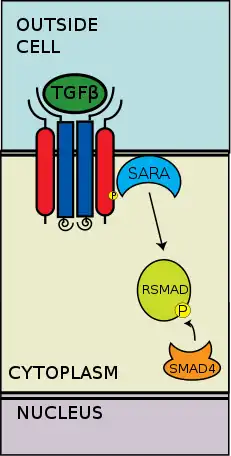
The extracellular type II and type I kinase receptors binding to the TGF-β ligands. Transforming growth factor-β (TGF-β) is a superfamily of cytokines that play a significant upstream role in regulating of morphogenesis, homeostasis, cell proliferation, and differentiation.[2] The significance of TGF-β is apparent with the human diseases that occur when TGF-β processes are disrupted, such as cancer, and skeletal, intestinal and cardiovascular diseases.[3][4] TGF-β is pleiotropic and multifunctional, meaning they are able to act on a wide variety of cell types.[5]
Mechanism
The effects of transforming growth factor-β (TGF-β) are determined by cellular context. There are three kinds of contextual factors that determine the shape the TGF-β response: the signal transduction components, the transcriptional cofactors and the epigenetic state of the cell. The different ligands and receptors of TGF-β are significant as well in the composition signal transduction pathway.[2]
- the signal transduction components: ligand isoforms, ligand traps, co-receptors, receptor sub-types, inhibitory SMAD proteins, crosstalk inputs
- the transcriptional cofactors of SMAD proteins: pluripotency factors, lineage regulators, DNA-binding cofactors, HATs and HDACs, SNF, chromatin readers
- the epigenetic factors: heterochromatin, pluripotency marks, lineage marks, EMT marks, iPS cell marks, oncogenic marks.
Upstream pathway
The type II receptors phosphorylate the type I receptors; the type I receptors are then enabled to phosphorylate cytoplasmic R-Smads, which then act as transcriptional regulators.[6][2] Signaling is initiated by the binding of TGF-β to its serine/threonine receptors. The serene/threonine receptors are the type II and type I receptors on the cell membrane. Binding of a TGF-β members induces assembly of a heterotetrameric complex of two type I and two type II receptors at the plasma membrane.[6] Individual members of the TGF-β family bind to a certain set of characteristic combination of these type I and type II receptors.[7] The type I receptors can be divided into two groups, which depends on the cytoplasmic R-Smads that they bind and phosphorylate. The first group of type I receptors (Alk1/2/3/6) bind and activate the R-Smads, Smad1/5/8. The second group of type I reactors (Alk4/5/7) act on the R-Smads, Smad2/3. The phosphorylated R-Smads then form complexes and the signals are funneled through two regulatory Smad (R-Smad) channels (Smad1/5/8 or Smad2/3).[6][2] After the ligand-receptor complexes phosphorylate the cytoplasmic R-Smads, the signal is then sent through Smad 1/5/8 or Smad 2/3. This leads to the downstream signal cascade and cellular gene targeting.[6][5]
Downstream pathway
TGF-β regulates multiple downstream processes and cellular functions. The pathway is highly variable based on cellular context. TGF-β downstream signaling cascade includes regulation of cell growth, cell proliferation, cell differentiation, and apoptosis.[8]
See also
References
- ↑ Miller DS, Schmierer B, Hill CS (July 2019). "TGF-β family ligands exhibit distinct signalling dynamics that are driven by receptor localisation". Journal of Cell Science. 132 (14): jcs234039. doi:10.1242/jcs.234039. PMC 6679586. PMID 31217285.
- 1 2 3 4 Massagué J (October 2012). "TGFβ signalling in context". Nature Reviews. Molecular Cell Biology. 13 (10): 616–30. doi:10.1038/nrm3434. PMC 4027049. PMID 22992590.
- ↑ Kashima R, Hata A (January 2018). "The role of TGF-β superfamily signaling in neurological disorders". Acta Biochimica et Biophysica Sinica. 50 (1): 106–120. doi:10.1093/abbs/gmx124. PMC 5846707. PMID 29190314.
- ↑ Huang T, Schor SL, Hinck AP (September 2014). "Biological activity differences between TGF-β1 and TGF-β3 correlate with differences in the rigidity and arrangement of their component monomers". Biochemistry. 53 (36): 5737–49. doi:10.1021/bi500647d. PMC 4165442. PMID 25153513.
- 1 2 Letterio JJ, Roberts AB (1998-04-01). "Regulation of immune responses by TGF-beta". Annual Review of Immunology. 16 (1): 137–61. doi:10.1146/annurev.immunol.16.1.137. PMID 9597127.
- 1 2 3 4 Vilar JM, Jansen R, Sander C (January 2006). "Signal processing in the TGF-beta superfamily ligand-receptor network". PLOS Computational Biology. 2 (1): e3. arXiv:q-bio/0509016. Bibcode:2006PLSCB...2....3V. doi:10.1371/journal.pcbi.0020003. PMC 1356091. PMID 16446785.
- ↑ Heldin CH, Moustakas A (August 2016). "Signaling Receptors for TGF-β Family Members". Cold Spring Harbor Perspectives in Biology. 8 (8): a022053. doi:10.1101/cshperspect.a022053. PMC 4968163. PMID 27481709.
- ↑ Li N, Xie C, Lu NH (2015). "Transforming growth factor-β: an important mediator in Helicobacter pylori-associated pathogenesis". Frontiers in Cellular and Infection Microbiology. 5: 77. doi:10.3389/fcimb.2015.00077. PMC 4632021. PMID 26583078.